
Fig. 1. E. popa popa
By Paul D. Brock
"Papillon", 40 Thorndike Road, Slough, SL21SR, England.
E-mail: Paul@pbrock40.fsnet.co.uk
| ! | Please note: this is not a primary source and does not exactly reproduce the original document. In particular, the layout will be very different to the original, due simply to the limitations of HTML as an information medium. However, you should also exercise caution with respect to the text presented here. In order to be reproduced here, the original paper has photocopied, scanned into a computer, OCRed (to turn the page image into text), and then hand corrected. Any or all of these steps could have introduced errors. If you would like to use the information herein for research purposes, you are strongly advised to view an original. Should you find any errors of reproduction, please let the maintainer of this web page know. | ! |
Brock, P. D. (2002), Studies on the Australasian stick-insect genus Extatosoma Gray (Phasmida: Phasmatidae: Tropoderinae: Extatosomatini), Journal of Orthoptera Research, Dec. 2001, 10(2): 303-313
This paper is Copyright © 2003 Paul D. Brock. It is reproduced here with the kind permission of the author (granted 15 May 2003) and the publisher (granted by Nancy Morris, 6 Jun 2003). If you wish to reproduce this paper in any form or medium, please contact Paul Brock for permission.
Abstract
| Page 303 |
Key words
Phasmatodea, Phasmatoptera, Cheleutoptera, Macleay's Spectre, Giant Prickly Stick Insect, Phasmidae
Introduction
One of the main taxonomic problems in the Phasmida is that variation can be extreme. Without examination of eggs and/or the rearing of a series of specimens, it is sometimes difficult to realize the extent of variation within individual species.
The large, spiny Extatosoma are one of the most spectacular genera of stick insects cultured. Specimens collected in north Queensland, Australia have been commonly reared since the 1960s and are often displayed in zoos and insect farms worldwide (Brock 2000). Specimens from various parts of southeast Queensland in Australia, Irian Jaya and Papua New Guinea have also recently been cultured. There have been a number of published papers on Extatosoma tiaratum, covering aspects such as behavior and biology. Gurney (1947) provided a useful synopsis of the genus, including a key to females, which I have updated in this paper. Carlberg (1987) includes useful notes on biology, with differences in development between cultures kept in Australia and Europe. Brock (1992) gives rearing notes and details/illustrations of defensive behavior.
Following the collection and rearing of similar Australian Extatosoma laying differently shaped eggs, there has recently been some doubt as to how many species were associated with that country (Beccaloni 1993, Brock 1999a). Further studies have uncovered wide variation within the single Extatosoma species now recognized as valid. The same remark applies to the only other Extatosoma species found in Irian Jaya and Papua New Guinea.
Details of the commoner leaf mimics and differently shaped lichen mimics are given in the keys. The downgrading of two Extatosoma species to new subspecies is proposed, and a lectotype designated for one species. Following a key to adults and eggs of the taxa, the species are presented with a full listing of synonyms beneath each valid species. For type material, which has been examined by me (except for paratypes of E. carlbergi in BPBM), details are given.
Abbreviations for Depositories
| AMSA | Australian Museum, Sydney, Australia |
| ANIC | Australian National Insect Collection, Canberra, Australia |
| BMNH | Natural History Museum, London, United Kingdom |
| BPBM | Bernice P. Bishop Museum, Hawaii, Honolulu, USA |
| MMUS | Macleay Museum, University of Sydney, Sydney, Australia |
| NHMW | Naturhistorisches Museum, Wien, Austria |
| OXUM | Oxford University Museum, Oxford, United Kingdom |
| RMNH | Nationaal Natauurhistorische Museum, Leiden, Netherlands |
| RSME | Royal Scottish Museum, Edinburgh, United Kingdom |
| ZMAN | Zoologisch Museum, Amsterdam, Netherlands |
| ZMUC | Zoologisk Museum, Copenhagen University, Denmark |
Key to adult females of Extatosoma
(Figs 1-4)
| 1. | Lateral expansions of abdominal segments 5-7 large and often overlapping. Segments 2-4 and 8-10 usually without lateral expansions. Generally green or brown; not mottled. Dorsal expansions of mid and hind femora broad, usually not arcuate (leaf mimicking insects) | 2 | |
| - | Lateral expansions of abdominal segments 5-7 small, non-overlapping. Segments 2-4 and 8-10 usually bearing lateral expansions. Body color green or brown, heavily white and black mottled. Dorsal expansions of mid and hind femora usually arcuate (lichen mimics) | 3 | |
| |||
| 2. | A conspicuous V-shaped pale mark nearly always present on mesonotum; metanotum and first abdominal segment each with a pair of erect, well developed lamellae. Dorsal lamellae of abdominal segments 5-6 each occupying about one-third length of segment, the base of each scarcely wider than apex and not extending in front of middle of segment; a compound lamellate spine on mesonotum between bases of tegmina. Body color pale to dark brown. Body length 116-150 mm. Distribution: Irian Jaya, Papua New Guinea | popa popa | |
| - | No V-shaped pale mark on mesonotum (or very faint); median lamellae of metanotum and first abdominal segment absent or weakly developed; spines separate or weakly confluent basally. Dorsal lamellae of abdominal segments 5-6 extending in front of middle of segment; the base of each lamella wider than apex; individual spines at base of mesonotum. Body color green or brown (rarely yellow). Body length 100-160 mm. Distribution in Australia: New South Wales, southeast and north Queensland | tiaratum tiaratum | |
| 3. | A conspicuous V-shaped pale mark nearly always present on mesonotum; metanotum and first abdominal segment each with a pair of erect, well developed lamellae. Dorsal lamellae of abdominal segments 5-6 each occupying about one-third length of segment, the base of each scarcely wider than apex and not extending in front of middle of segment; a compound lamellate spine on mesonotum between bases of tegmina. Body length 116-165 mm. Distribution: Irian Jaya, Papua New Guinea | popa carlbergi | |
| - | No V-shaped pale mark on mesonotum (or very faint); median lamellae of metanotum and first abdominal segment absent or weakly developed; spines separate or weakly confluent basally. Dorsal lamellae of abdominal segments 5-6 extending in front of middle of segment; the base of each lamella wider than the apex; individual spines at base of mesonotum. Body length 120-130 mm. Distribution in Australia: New South Wales, Lord Howe Island, southeast and north Queensland | tiaratum bufonium | |

Fig. 1. E. popa popa
![]() .
.
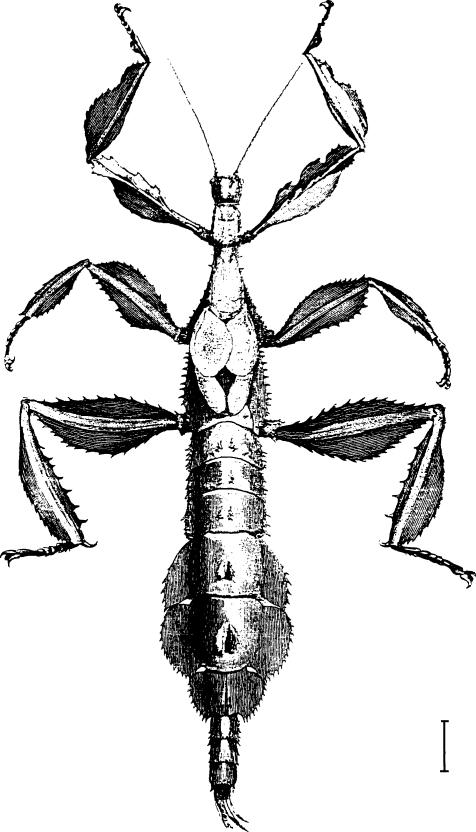
Fig. 2. E. tiaratum tiaratum,
![]() holotype,
Australia (scale: 10 mm); almost certainly from Parramatta, New South
Wales (reproduced from Macleay 1826).
holotype,
Australia (scale: 10 mm); almost certainly from Parramatta, New South
Wales (reproduced from Macleay 1826).
| Page 305 |

Fig. 3. E. popa carlbergi
![]()
Key to adult males of Extatosoma
(Figs 5-6)
| 1. | Hindwings plain, with only outer margin dark. White V-shaped mark on mesonotum. Body length 82-91 mm | popa |
| - | Hindwings chequered; dark brown/blackish with whitish bands. No white V-shaped mark on mesonotum. | 2 |
| 2. | Body length 81-115 mm. Distribution: New South Wales, Lord Howe Island, southeast and north Queensland | tiaratum |
Note: males of lichen mimics do not differ from normal leaf mimics.
Key to eggs of Extatosoma
(Figs 7-9)
| 1. | Micropylar plate with conspicuous lateral arm either side of the micropylar cup area. Capsule length 5.06-5.31 mm, height 4.42-4.5 6 mm, width 3.71 -3.84 mm | popa |
| - | Micropylar plate lacking conspicuous lateral arm either side of the micropylar cup area. Capsule length 4.5 mm or less | 2 |
| 2. | Capsule slightly glossy; with brown-pointed capitulum. Micropylar plate reaching operculum rim, where it is expanded. Capsule length 3.3-4.2 mm, height 3.5 mm, width 2.9-3.2 mm. Distribution in Australia: New South Wales, southeast Queensland | tiaratum |
| - | Capsule on average larger, glossy; with broad, whitish capitulum. Micropylar plate reaching operculum rim, where it is slightly expanded. Capsule length 3.7-4.5 mm, height 3.5-4.2 mm, width 2.8-3.1 mm. Distribution: in Australia: north Queensland | tiaratum |
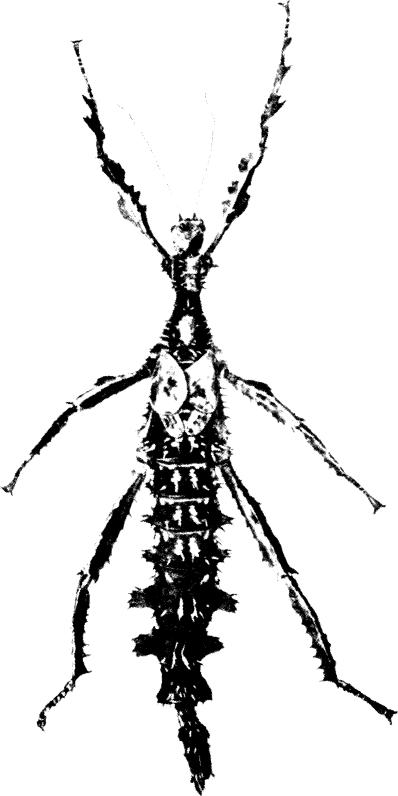
Fig. 4. E. tiaratum bufonium
![]() (after Gurney 1947).
(after Gurney 1947).
| Page 306 |
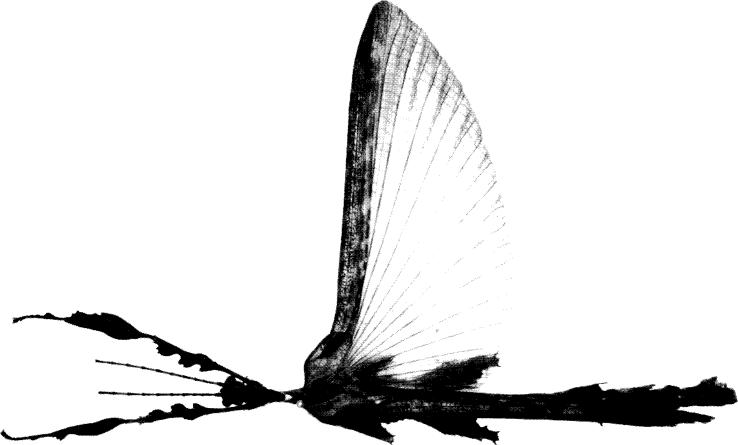
Fig. 5. Extatosoma popa popa
![]()

Fig. 6. Extatosoma tiaratum tiaratum
![]()
| Page 307 |
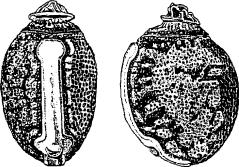
| 7. |  |
8. |  |
9. | 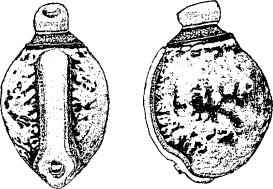 |
| Figs 7-9. Dorsal and lateral views of eggs: 7. E. popa Papua New Guinea (same as E. caribergi). 8. E. tiaratum bufonium Australia: Mt. Nebo, southeast Queensland (same as E. tiaratum tiaratum from southeast Queensland or NSW). 9. E. tiaratum tiaratum Australia: north Queensland. (Scale: 1 mm.) | |||||
Notes: eggs laid by leaf mimics and lichen mimics within each species are identical. Egg coloration is very variable and no two eggs are exactly alike. The capitular cap shrivels with age and may disappear entirely. Smaller eggs, which often do not hatch, may be parthenogenetic, or are laid by smaller-sized females; if they do hatch, development is much slower than if eggs are fertilized.
Key to newly-hatched nymphs of Extatosoma
(Fig. 10)
| 1 | Largely brown or black, but head either red or brown; legs mottled in different shades. Sometimes with white mark on mesonotum | 2 |
| - | Completely black, except for a conspicuous white V-shaped mark on mesonotum | popa |
| 2 | Head dark brown. Body light orange/brown; abdomen dark brown/black. Distribution in Australia: New South Wales, southeast Queensland | tiaratum |
| - | Head red or dark brown. Body dark brown or black (if dark brown, abdomen black). Distribution in Australia: north Queensland | tiaratum |
Within days, nymphs which have been feeding change color. For example, in E. tiaratum from north Queensland the red heads become dark brown, along with the body; E. tiaratum from New South Wales and southeast Queensland darken up and resemble the north Queensland insects. [Note: for color illustrations of 1st instar nymphs see Brock 2001].
Extatosoma Gray 1833: 23.
Type species. - Phasma tiaratum Macleay 1826: 455, by
subsequent designation of Kirby 1904: 380 (after
Burmeister 1838 listed E. hopei as a synonym of E. tiaratum,
there was only one Extatosoma species 1838 to 1873).
Ectatosoma Gray 1835: 29 (incorrect
subsequent spelling).
Ectatosoma Gray;
Brull, 1836: 113 (incorrectly listed as a synonym of Tropidoderus
Gray
1835).
Characteristics of the genus. - Large; body broad and spinose in female, slenderer and less spinose in male. The male is winged, female with rudimentarywings. Head prognathous, dorsal apex conical and spinose. Three ocelli distinct in male, lacking in female. Antennae simple, of moderate length (much longer in male), pubescent. Mesonotum dilated in female, not twice length of pronotum. Forewings shortened. In male, oval, as long as metanotum; in female, broader, but little over half length of metathorax. Hindwings of male large, reaching to around apex of abdomen. In female shrivelled and rudimentary (shorter than forewings). Abdominal segments greatly expanded laterally, particularly 5th-7th segments. In female, paired median lamellae present on abdominal segments. Legs moderately long to short; femora and tibiae spinose, trigonate, broadly dilated, particularly in female. Mid and hind tibiae with apical hooked spine. Female with large boat-shaped operculum extending well beyond end of abdomen; valves long, filamentous, apically curved. Subgenital plate in male boat-shaped. End of abdomen a closed tube. The large eggs are very conspicuous. Capsule oval, with broad, slightly raised micropylar plate, extending full length of dorsal surface. The eggs were first described by Kaup (1871), who figured the egg of Extatosoma tiaratum. Although later authors have not remarked on the clearly different appearance of E. tiaratum eggs figured, the best descriptions are by Korboot 1961 (New South Wales/southeast Queensland) and by Heather 1965. Clark 1976 covers E. tiaratum from north Queensland, also illustrated by Key 1970. Sellick (1997) established the monotypic tribe Extatosomatini and figured a normal and an abnormal egg.
Extatosoma tiaratum tiaratum (Macleay)
(Figs 2, 6, 9)
Phasma tiaratum Macleay 1826:
455, pl. Tab. B, Figs 3, 4. Holotype
![]() , AUSTRALIA*
(believed lost, not traced in MMUS).
, AUSTRALIA*
(believed lost, not traced in MMUS).
* Described in Appendix B of King's survey. The insects collected during Captain King's voyages came from all parts of the Australian coast; no locality specified. However Gray (1833) stated that Allan Cunningham (a botanist who collected on King's voyages) informed him “they are found on
| Page 309 |
Distribution. - Widespread in parts of New South Wales, southeast and north Queensland. Historically reported from Tasmania (and possibly Victoria), but records considered to be in error (Froggatt 1922). Old records from New Guinea are repeated from de Haan 1842, where his "var." was later described as E. popa. Specimens occur at various altitudes, with the lichen mimics often found at higher altitudes.
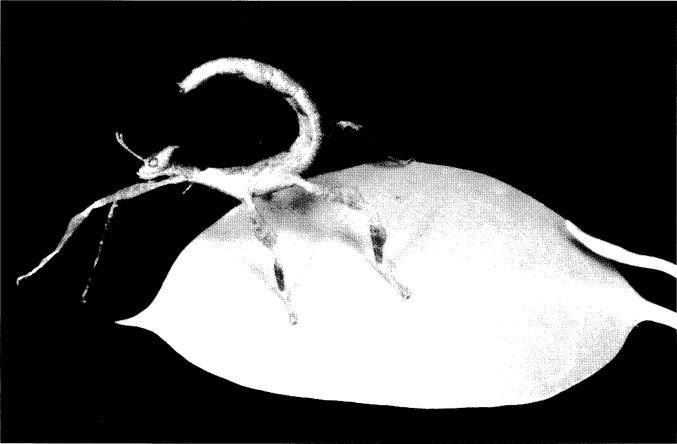
Fig. 10. Young nymph of lichen mimic Extatosoma tiaratum
bufonium, which has been eating and is close to moulting. This is
the characteristic posture of all Extatosoma nymphs, with the
abdomen curled over the body.
There is a possibility that further studies will reveal that the north Queensland insects are distinct from those from New South Wales and southeast Queensland. Whilst eggs and first instar nymphs differ (see key above), it is conceivable that these insects have evolved to closely match local ants. It is known that ants are attracted to the capitulum of phasmid eggs, and the eggs then carried to the nest; consequently buried eggs suffer reduced rates of parasitism by wasps (Hughes & Westoby 1992). See Windsor et al. (1996) for a review of the literature.

Fig. 11. Extatosoma tiaratum bufonium lichen mimic
![]() (same as tiaratum tiaratum).
(same as tiaratum tiaratum).
Extatosoma tiaratum bufonium Westwood stat. n.
(Figs 4, 8, 10, 11)
Extatosoma bufonium Westwood 1874:
174. Holotype
![]() nymph, AUSTRALIA (OXUM)
nymph, AUSTRALIA (OXUM)
Extatosoma (?) bufonium Westwood; Kirby 1904: 381.
Extatosoma bufonium Westwood;
Redtenbacher 1908: 381.
Extatosoma elongatum Froggatt,
1922: 345. Lectotype
![]() , AUSTRALIA: Gosford, New
South Wales, 1921, leg. W. W. Froggatt (ANIC), here designated.
Paralectotype
, AUSTRALIA: Gosford, New
South Wales, 1921, leg. W. W. Froggatt (ANIC), here designated.
Paralectotype
![]() , AUSTRALIA: Camden, New South Wales
(MMUS). Listed as a synonym of E. bufonium by Vickery 1983:
7 (confirmed).
, AUSTRALIA: Camden, New South Wales
(MMUS). Listed as a synonym of E. bufonium by Vickery 1983:
7 (confirmed).
Distribution. - Widespread in parts of New South Wales and southeast Queensland and occasionally reported in the Atherton area, north Queensland. In the majority of cases, these localities are high altitude, which seldom overlap with tiaratum tiaratum. There is a old museum specimen collected from Lord Howe Island, New South Wales.
Extatosoma popa popa Stål
(Figs 1, 5, 7, 12)
Extatosoma popa
Stål 1875: 84. Holotype
![]() PAPUA NEW GUINEA (RMNH) based on de Haan 1842 - partial
misidentification of E. tiaratum. See p. 110, pl. 10: 2,
PAPUA NEW GUINEA (RMNH) based on de Haan 1842 - partial
misidentification of E. tiaratum. See p. 110, pl. 10: 2,
Ectatosoma popa Stål;
Redtenbacher 1908: 381.
Distribution. - Widespread in Irian Jaya and Papua New Guinea. Beccaloni (1993) pointed out that E. p. carlbergi mainly occurs at higher altitudes (1100-1600m) than E. p. popa (to 1200m). In 1999 Herwaarden (pers. comm.) found specimens of both “forms” feeding on Casuarina, between Kelila and Bokondini, Central Mountain Range, Irian Jaya (1500m), indicating that leaf mimics can also occur at high altitudes.
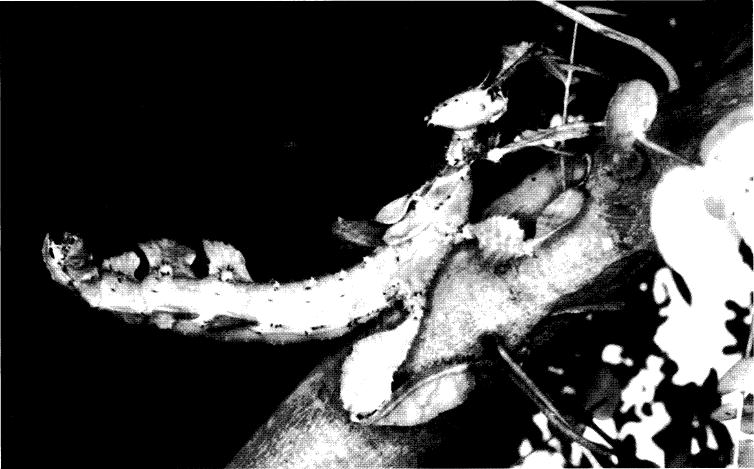
Fig. 12. Extatosoma popa popa normal leaf mimic
![]() , but with gaps between lateral expansions of abdominal segments 5-7.
, but with gaps between lateral expansions of abdominal segments 5-7.
| Page 310 |
Extatosoma popa carlbergi Beccaloni stat. n.
(Fig. 3)
Extatosoma carlbergi
Beccaloni 1993: 115. Holotype
![]() , PAPUA NEW GUINEA: Wau, Morobe Province, golf course, c. 1150 m,
ex. Calliandra surinamensis, 14.vii.1990, G. W. Beccaloni (BMNH).
Paratype series PAPUA NEW GUINEA: 2
, PAPUA NEW GUINEA: Wau, Morobe Province, golf course, c. 1150 m,
ex. Calliandra surinamensis, 14.vii.1990, G. W. Beccaloni (BMNH).
Paratype series PAPUA NEW GUINEA: 2
![]()
![]() ,
Tari, Southern Highlands Province, 1600 m, ex. Casuarina, 4.x.1963,
R. Straatman (BPBM);
,
Tari, Southern Highlands Province, 1600 m, ex. Casuarina, 4.x.1963,
R. Straatman (BPBM);
![]() damaged, Karimui, Chimbu Province,
4.vi.1961, Gressitt (BPBM);
damaged, Karimui, Chimbu Province,
4.vi.1961, Gressitt (BPBM);
![]() Wantoat, Morobe Province,
1.xii.1957, ex. Casuarina, leg. R. W. Paine (BMNH).
Wantoat, Morobe Province,
1.xii.1957, ex. Casuarina, leg. R. W. Paine (BMNH).
Distribution. - Widespread in Irian Jaya and Papua New Guinea. This subspecies mainly occurs at higher altitude (1100-1600 m) than E. papa papa, although there is occasionally an overlap.
Non-type material examined. - Selected material examined in detail (measurements recorded) is listed below. In addition, reared material and specimens deposited in many museums in Europe and Australia have been examined.
E. tiaratum tiaratum. - Normal specimens; leaf mimics. All
from southeast Queensland, AUSTRALIA:
![]()
![]() , Brisbane (ZMUC);
, Brisbane (ZMUC);
![]() , Taylor Range, Brisbane, L. D., Rothschild Bequest 1939;
, Taylor Range, Brisbane, L. D., Rothschild Bequest 1939;
![]() , Moreton Bay (BMNH);
, Moreton Bay (BMNH);
![]()
![]() , Mt. Glorius 635 m, (
, Mt. Glorius 635 m, (
![]() 10.i.1981,
10.i.1981,
![]() 22.i.1985), T. Hiller;
22.i.1985), T. Hiller;
![]() , Mt. Nebo, Ironbok, v.1985, T. Hitler;
, Mt. Nebo, Ironbok, v.1985, T. Hitler;
![]() Mt. Nebo, 500m on Eucalyptus, iii.1984, S. Wilson,
28.vii. 1985 (all coll. T. Hitler);
2
Mt. Nebo, 500m on Eucalyptus, iii.1984, S. Wilson,
28.vii. 1985 (all coll. T. Hitler);
2
![]()
![]() , AUSTRALIA (OXUM - specimens
discussed by Gray, 1833, most probably from Parramatta, NSW).
From north Queensland, AUSTRALIA:
, AUSTRALIA (OXUM - specimens
discussed by Gray, 1833, most probably from Parramatta, NSW).
From north Queensland, AUSTRALIA:
![]() , Mossman (RSME);
, Mossman (RSME);
![]() , north Queensland, 1967, C. R. Hembrow;
2
, north Queensland, 1967, C. R. Hembrow;
2
![]()
![]() , Geraldton, Meek, Rothschild Bequest
1939) (BMNH);
, Geraldton, Meek, Rothschild Bequest
1939) (BMNH);
![]() , ex. Garradunga, reared, 16.1.1994;
, ex. Garradunga, reared, 16.1.1994;
![]() , 17.28S 146.01E, 2km E. of Garradunga, 2.iv.1991, G. Milledge;
, 17.28S 146.01E, 2km E. of Garradunga, 2.iv.1991, G. Milledge;
![]() , 17.26S 146.00E 15 km NNW of Innisfail, 17.ii.1989, G. Milledge;
, 17.26S 146.00E 15 km NNW of Innisfail, 17.ii.1989, G. Milledge;
![]() , reared, M. Lazenbv, i.1994 (all coll. P. D. Brock).
, reared, M. Lazenbv, i.1994 (all coll. P. D. Brock).
E. tiaratuni bufonium. - Lichen mimics,
formerly known as E. bufonium.
All from AUSTRALIA - either southeast Queensland (Q) or New South Wales (NSW):
![]() , Lord Howe Island, NSW, probably late 1800s (NHMW);
, Lord Howe Island, NSW, probably late 1800s (NHMW);
![]() nymph, Moreton Bay (= Brisbane area), Q (BMNH);
nymph, Moreton Bay (= Brisbane area), Q (BMNH);
![]() nymph,
nymph,
![]() nymph, ex. Mt. Nebo, Q,
reared on Eucalyptus, x.1998 (coll. P. D. Brock);
nymph, ex. Mt. Nebo, Q,
reared on Eucalyptus, x.1998 (coll. P. D. Brock);
![]() , Mt. Nebo, Q, 23.vii.1997 (died x.1997), leg.
T. Hiller;
2
, Mt. Nebo, Q, 23.vii.1997 (died x.1997), leg.
T. Hiller;
2
![]()
![]() , Wilsons, NSW, 2.iii.1925,
ex. coll. E. E. Adams (all coll. T. Hiller).
From north Queensland:
, Wilsons, NSW, 2.iii.1925,
ex. coll. E. E. Adams (all coll. T. Hiller).
From north Queensland:
![]() nymph, Atherton, 3-18.xi.1972, A. M. Hemmingsen (ZMUC).
nymph, Atherton, 3-18.xi.1972, A. M. Hemmingsen (ZMUC).
![]() , Narara via Gosford, NSW, 27.v.1962, H. S. Cropper;
, Narara via Gosford, NSW, 27.v.1962, H. S. Cropper;
![]() , Craven via West Maitland, NSW, 25.ii. 1932, H. M. Williams;
, Craven via West Maitland, NSW, 25.ii. 1932, H. M. Williams;
![]() , Kendal, NSW, 2.vi.1936, E. Welling;
, Kendal, NSW, 2.vi.1936, E. Welling;
![]() , Yarramalong, NSW, xii.1928, Mrs K. Mayo;
, Yarramalong, NSW, xii.1928, Mrs K. Mayo;
![]() , Tumbiumba nr Gosford, NSW, iii.1955, Mrs E. Wilson;
, Tumbiumba nr Gosford, NSW, iii.1955, Mrs E. Wilson;
![]() , Bow, NSW, 27.v.1962, H. S. Cropper;
, Bow, NSW, 27.v.1962, H. S. Cropper;
![]() , Bowraville, NSW, 10.ii.1932, Dr A. C. Burstal;
, Bowraville, NSW, 10.ii.1932, Dr A. C. Burstal;
![]() , Wauchope, NSW, iii.1937, C. B. Lane;
, Wauchope, NSW, iii.1937, C. B. Lane;
![]() , Dungog, NSW, 9.iii.1935, Dungog Chronicle;
, Dungog, NSW, 9.iii.1935, Dungog Chronicle;
![]() , Turtable Falls nr Nimbin, NSW, xi.1951, on Persimmon
tree, R. Heading;
, Turtable Falls nr Nimbin, NSW, xi.1951, on Persimmon
tree, R. Heading;
![]() Wootton via Bulladelah, NSW, xi.1948, L. Love.
There are also 3
Wootton via Bulladelah, NSW, xi.1948, L. Love.
There are also 3
![]()
![]() marked as
bufonium, which may belong to this species:
marked as
bufonium, which may belong to this species:
![]() , Macleay River, NSW, vi.1948, Mrs Mary Hawes;
, Macleay River, NSW, vi.1948, Mrs Mary Hawes;
![]() , Macksville, NSW, Miss Scurr, K54567;
, Macksville, NSW, Miss Scurr, K54567;
![]() , Sydney, NSW, K13257 (AMSA)
, Sydney, NSW, K13257 (AMSA)
F. papa popa. - Normal specimens; leaf mimics.
All from PAPUA NEW GUINEA (PNC) or IRIAN JAYA (IJ).
![]() , IJ, Sorong, 3.x.1959, leg. M. F. Kleiman-Eves (ZMAN);
, IJ, Sorong, 3.x.1959, leg. M. F. Kleiman-Eves (ZMAN);
![]() , PNG, Owgarra, purchased Janson 1906;
, PNG, Owgarra, purchased Janson 1906;
![]() , PNG, Aroa R., Meek, Rothschild Bequest 1 939;
, PNG, Aroa R., Meek, Rothschild Bequest 1 939;
![]() , PNG, Madang District, Finisterre Mts, Budemu,
C. 4000 ft, i5-24.x.1964, M. E. Bacchus;
, PNG, Madang District, Finisterre Mts, Budemu,
C. 4000 ft, i5-24.x.1964, M. E. Bacchus;
![]() nymph, PNC, Madang District, Finisterre Mts, Darmanti,
3550 ft, 2-11.x.1964, M. E. Bacchus;
nymph, PNC, Madang District, Finisterre Mts, Darmanti,
3550 ft, 2-11.x.1964, M. E. Bacchus;
![]() , PNG, Sharp collection, acquired 1905;
, PNG, Sharp collection, acquired 1905;
![]() , IJ, 140°E long. 3°10S, 300-600 iii, W. Stüber;
3
, IJ, 140°E long. 3°10S, 300-600 iii, W. Stüber;
3
![]()
![]() ,
,
![]() nymph, PNG, Wau,
Morobe Province, on golfcourse, 1 150 iii, 13.vii.1990, C. W. Beccaloni;
7
nymph, PNG, Wau,
Morobe Province, on golfcourse, 1 150 iii, 13.vii.1990, C. W. Beccaloni;
7
![]()
![]() , 2
, 2
![]()
![]() ,
PNG, ex. Wau, all reared ix.1978 from specimens collected
by A. J. E. Harman in x.1977 (all BMNH);
,
PNG, ex. Wau, all reared ix.1978 from specimens collected
by A. J. E. Harman in x.1977 (all BMNH);
![]()
![]() , PNG, Kerowagi, Chimbu Province,
iv.1991 (
, PNG, Kerowagi, Chimbu Province,
iv.1991 (
![]() 20.xi.1978);
20.xi.1978);
![]() , IJ, Nabire, Pantai District, 1996;
, IJ, Nabire, Pantai District, 1996;
![]() , ex PNG, bred by A. J. E. Harman, 20.xii.1998;
, ex PNG, bred by A. J. E. Harman, 20.xii.1998;
![]()
![]() , PNG, Menyamya, Morobe Province,
iii.1995 (all coll. P. D. Brock);
3
, PNG, Menyamya, Morobe Province,
iii.1995 (all coll. P. D. Brock);
3
![]()
![]() , IJ, between Kelila and Bokondini,
Central Mountain Range, 1500 m, 6.x.1999, H. C. M. van Herwaarden
& O. van Gorkom (coll. H. C. M. van Herwaarden).
, IJ, between Kelila and Bokondini,
Central Mountain Range, 1500 m, 6.x.1999, H. C. M. van Herwaarden
& O. van Gorkom (coll. H. C. M. van Herwaarden).
Note: it is not known how many of the
![]()
![]() listed above are linked with lichen mimics, as specimens would be
indistinguishable.
listed above are linked with lichen mimics, as specimens would be
indistinguishable.
E. papa carlbergi. -
lichen mimics, formerly known as E. carlbergi.
From OAPUA NEW GUINEA (PNG) or IRIAN JAYA (IJ).
![]() PNG, Watut, Wau Valley, Morobe Province, 1100 m, 1992;
PNG, Watut, Wau Valley, Morobe Province, 1100 m, 1992;
![]() PNG, Menvamva, iv. 1995;
PNG, Menvamva, iv. 1995;
![]() nymph, PNC, Waria Valley, Morobe Province, iii. 1978,
1000 m (coll. P. D. Brock);
3
nymph, PNC, Waria Valley, Morobe Province, iii. 1978,
1000 m (coll. P. D. Brock);
3
![]()
![]() IJ, between Kelila and Bokondini,
Central Mountain Ranger 1500 m, 6.x.1999, H. C. M. van Herwaardcn
& O. van Gorkom (coll. H. C. M. van Herwaarden).
IJ, between Kelila and Bokondini,
Central Mountain Ranger 1500 m, 6.x.1999, H. C. M. van Herwaardcn
& O. van Gorkom (coll. H. C. M. van Herwaarden).
First description of the male of Extatosoma papa from Papua New Guinea. - Dark brown insect, variably mottled with leaf-like expansions on legs. Hindwings plain, with outer margin brown. Body length 82-91 mm.
Head: conical, with strong trigonal spine each side of occipital crest; otherwise a few tubercles or small spines close to cluster of spines. Three ocelli. Antennae long, but not quite reaching end of forelegs.
Thorax: pronotum shorter than head, with short spines, longest pair towards posterior of segment. Mesonotum short, 1.3 times length of pronotum. Front of mesonotum with white V-shaped mark, surrounding black collar which ends with a pair of long spines, otherwise only a few small tubercles present. Metanotum slightly tuberculate, longer than mesonotum. Metasternum strongly spined.
Wings: forewings leaf-like; same color as body, but sometimes outer half darker brown. Hindwings long, exceeding
| Page 311 |
Abdomen: slender, segments 5-7 with well developed lateral expansions, with a slight gap between segments. Subgenital plate broad, end rounded with a deep central incision. When viewed laterally, last third raised, with central lobe. End of anal segment triangularly produced ventroposteriorly. Cerci slender, rounded at tip.
Legs: lateral leaf-like dentate expansions developed on all tibiae and femora. Those on fore femora split into three lobes. Fore tarsi with distinct basal lobe.
Measurements (mm): body length 82-91, Head 6-7, antennae 39-42, pronotum 4-5, mesonotum 6-7, metanotum 9-10, median segment 4.5-5. Forewings 15-16, hindwings 68-73. Femora: fore 19-20, mid 14-15, hind 18-19. Tibiae: fore 15-16.5, mid 11-12, hind 17-18. Cerci 1-1.5.
Notes on variation within species. - (Fig. 12) There are several ‘variations’ within both Extatosoma species. Firstly, size: the length of specimens varies considerably, both in the wild and in culture. E. popa is usually longer than E. tiaratum, although some giants have been reported for the latter species. The longest I know of is a 160 mm female from Kuranda, north Queensland, which was not preserved. I have reared a 150 mm specimen. There is some evidence that females of E. tiaratum from north Queensland are longer than those from New South Wales and southeast Queensland. The size difference is, perhaps, not surprising in view of a larger egg capsule in addition to a hotter climate and different foodplants in some cases. However, even in north Queensland much smaller specimens around 100 mm occur. There does not appear to be much difference in average size between the lichen mimics and normal leaf mimics, although, once again, individual specimens can vary considerably in length.
Color is another very variable factor. No two eggs are exactly alike in color. Adults are often a similar shade of brown. With tiaratum, in the wild there are more green female specimens than occur in culture stocks; foodplant is probably a major factor in this. Although captive-bred stocks of males are nearly always brown, in north Queensland males around Garradunga range from dark brown, to grey, pale green, and orange (Jack Hasenpusch, pers. com.) In the same locality, mottled females have occasionally been reared, apparently resembling lichens (but still keying out to tiaratum tiaratum); an example is illustrated in Breedon (1995), but this specimen and others changed color to normal when kept in different conditions. The true lichen mimics also vary considerably in degree of mottling, and it is difficult to find two specimens exactly alike. Some other research has been undertaken on color changes (Korboot 1961), where light-adapted insects are yellow or green, dark-adapted insects orange or brown; rhythmic color change occurred diurnally: dark at night, light in the morning.
The number and size of body and abdominal spines varies, particularly in females, although the key features discussed earlier are consistent. Taking E. popa popa as an example, the foliose expansions on abdominal segments 5-7 may vary considerably in length and number. Whilst these lateral expansions usually overlap, sometimes they have gaps between them, although not to the same degree as lichen mimics (Fig. 12). The legs also vary; some specimens have more arcuate dorsal expansions of the femora than others. Although a generally reliable key feature to distinguish between E. popa and E. tiaratum, I have an E. popa caribergi female from Watut, Wau Valley lacking a V-shaped pale mark on its mesonotum.
The first records of gynandromorphism in tiaratum were reported by Rumbucher 1974. One of the two gynandromorph specimens reared was a halved gynandromorph, the other a mixture of male and female characteristics, with large hindwings as in the male. Carlberg (1981) gave details of a gynandromorph with mainly male characteristics. No gynandromorphs have yet been reported in E. popa. Although there seldom appears to be an overlap of the commoner leaf mimics and lichen mimics, several records are known, as follows: E. tiaratum tiaratum and E. tiaratum bufonium in Australia: Gosford, New South Wales; Mt. Nebo, south-east Queensland; Atherton, north Queensland. E. popa popa and E. popa carlbergi overlap in Papua New Guinea: Wau, Morobe Province and nearby Menyamya. In Irian Jaya, between Kelila and Bokondini, Central Mountain Range, 1500 m. So far, collectors have located the lichen mimics at higher altitudes, where leaf mimics are seldom found. The leaf mimics are normally strongly represented at lower altitudes, in gardens and in the bush.
Conclusion
Although E. tiaratum from north Queensland has been reared since the 1960s, until recently no comment was made on clear egg differences in comparison with eggs from southeast Queensland and New South Wales cultures.
The range of E. tiaratum shows a large gap in central Queensland where there are no records of this species (Fig. 13). In view of differences between the eggs and first instar nymphs of culture stocks from north Queensland, compared with other localities, it cannot be ruled out that they may at least represent different subspecies, albeit the adults are identical. It may take some time to research these aspects, by attempting crosses between culture stocks and analyzing the results, in addition to undertaking genetic studies. There is scope to make important studies in the wild, such as reviewing habitats, particularly as the north Queensland specimens may have evolved to more closely resemble ants in the area.
The degree of variation between leaf mimics and lichen mimics is wide within both species. However, each subspecies is usually consistent in shape, if not in color. Other phasmids use different colors to better match their surroundings, but rarely occur in a significantly different “form” as seen in Extatosoma. It is possible that this strategy is used to help ensure survival, on the basis that predators may learn to distinguish leaf mimics, but not lichen mimics, or vice versa. This is the view put forward by H. van Herwaarden (pers. com.), following observations in the field in Irian Jaya on E. popa popa and E. popa carlbergi, but I am not convinced
| Page 312 |
During my research, it became apparent that the majority of lichen mimics are found at higher altitudes, typically where the rainforest has a higher species density of ferns, mosses and lichens. This may account for the scarcity of records of lichen mimics in some cases, particularly in north Queensland where leaf mimic females are often found at low altitudes. This supports Beccaloni's views (1993), who noticed that the mottled patterns of lichen mimics resemble foliose epiphytic lichens such as Usnea, or leaves covered with epiphyllous growth. Whilst it is possible that lichen mimics have been overlooked by collectors searching at lower altitudes, this appears to be unlikely.
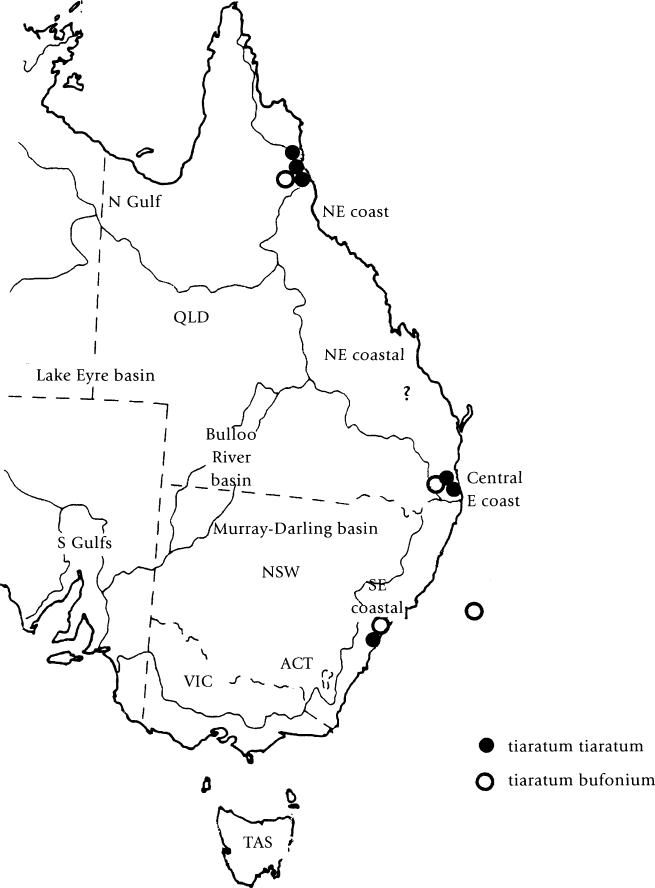
Fig. 13. Distribution of Extatosoma in Australia.
Acknowledgements
I wish to thank the following contacts for assistance in providing specimens or observations: S. Fellenberg (Sydney, New South Wales), J. Hasenpusch (Australian Insect Farm, Garradunga, Nr. Innisfail, Queensland), H. van Herwaarden (Goirle, Netherlands), T. Hiller (Mt. Glorious, Queensland), M. Humphrey (Macleay Museum, Sydney, New South Wales), J. Marshall (Natural History Museum, London, UK), G. Milledge, M. Moulds (Australian Museum, Sydney), D. Rentz (CSIRO, Canberra). Ronald Baxter (Ilford, UK) kindly permitted reproduction of egg sketches commissioned for his forthcoming book. Curators of several museums kindly allowed access to the collections.
Literature cited
Audinet-Serville J. G. 1838. Histoire Naturelles des Insectes. Orthoptères. Librarie Encyclopédique de Roret, Paris.
Baxter R. N. (in press). Rearing Stick and Leaf Insects. Chudleigh Publishing, Essex, UK.
Beccaloni G. W. 1993. A new species of Extatosoma Gray (Phasmatodea: Phasmatidae) from Papua New Guinea, with remarks on the species in the genus. Tijdschriftvoor Entomologie 136: 113-123.
Breedon, S. 1995. Paul and the Rainforest. Steve Parish Publishing Pty Ltd., Queensland.
Brock, P. D. 1992. Rearing and Studying Stick and Leaf-Insects. The Amateur Entomologists' Society, Feitham. The Amateur Entomologist Vol. 22.
Brock P. D. 1999a. The Amazing World of Stick and Leaf-Insects. The Amateur Entomologists' Society, Orpington. The Amateur Entomologist Vol. 26.
Brock P. D. 1999b. Stick and Leaf Insects of Peninsular Malaysia and Singapore. Malaysian Nature Society, Kuala Lumpur.
Brock P. D. 2000. A Complete Guide to Breeding Stick and Leaf Insects. T. F. H. Kingdom, Havant.
Brock P. D. 2001. Insect Gallery (photographs). Insecta 1: 24-25.
Brull A. M. 1836. Histoire naturelle des Insectes. IX. Orth. Paris.
Burmeister H. 1838. Handbuch der Entomologie. Vol. 2. T. C. F. Enslin, Berlin.
Carlberg U. 1981. A Gynandromorph specimen of Extatosoma tiaratum (MacLeay) (Insecta: Phasmida). Zoologischer Anzeiger, Jena 207: 320-322.
Carlberg U. 1987. Culturing stick- and leaf-insects (Phasmida) - A review. Z. Versuchstierkd. 29: 39-63.
Clark J. T. 1976. The eggs of stick insects (Phasmida): a review with descriptions of the eggs of eleven species. Systematic Entomology 1: 95-105.
Froggatt W. W. 1922. Description of a new phasma belonging to the genus Extatosoma. Proceedings of the Linnean Society N. S. W. 47: 344-345.
Gray G. R. 1832. In Griffith, E. The Animal Kingdom, arranged in conformity with its organization by the Baron Cuvier. Insecta, Vol. 2, Whittaker, Treacher, and Co. London.
Gray G. R. 1833. The Entomology of Australia in a series of Monographs. Part 1. The monograph of the genus Phasma. Longman & Co, London.
Gray G. R. 1835. Synopsis of the Species of Insects Belonging to the Family of Phasmidae. Longman, Rees, Orme, Brown, Green and Longman, London.
Gurney A. B. 1947. Notes on some remarkable Australasian walkingsticks, including a synopsis of the genus Extatosoma (Orthoptera: Phasmatidae). Annals of the Entomological Society of America 40(3): 373-396.
Haan W. de 1842. Bijdragen tot de kennis der Orthoptera. In Temminck, C. L. Verhandelingen over de natuurlijke Geschiedenis der Nederlandsche overzeesche Bezittingen. Vol. 2. Leiden pp. 45-248, pl. 10-23.
Heather N. W. 1965. Studies on female genitalia of Queensland Phasmida. Journal of the Entomological Society of Queensland 4:33-38.
Hughes L. & Westoby, M. 1992. Capitula on stick insect eggs and elaiosomes on seeds: convergent adaptations for burial by ants. Functional Ecology 6: 642-648.
Kaup J. J. 1871. Ueber die Eier der Phasmiden. Berliner Entomologische Zeitschrift 15: 17-24, pl. 1.
Key K. H. L. 1970. Phasmatodea (Stick-insects). In: The Insects of Australia (CSIRO Division of Entomology), Pp. 348-359. Melbourne University Press, Melbourne.
Kirby W. F. 1904. A Synonymic Catalogue of Orthoptera. Vol. 1, Orthoptera, Euplexoptera, Cursoria, et Gressoria (Forficulidae, Hemimeridae, Blattidae, Mantidae, Phasmidae). Longmans & Co, London.
Korboot K. 1961. Observations on the Life Histories of the Stick Insects Acrophylla tessellata Gray and Extatosoma tiaratum Macleay. University of Queensland Papers 1: 16 1-169.
Macleay W. S. 1826. Annuloso, Catalogue of Insects, collected by Captain King, R. N. In: Captain Phillip P. King, Narrative of a survey of the Intertropical, and Western Coasts of Australia performed between the years 1818 and 1822. Vol 2., appendix B, Pp. 438-469, Table B.
McKeown K. C. 1940. Australian Insects. VIII. Orthoptera: 3. Stick and Leaf Insects. The Australian Museum Magazine 125-130.
| Page 313 |
Redtenbacher J. 1908. In Die Insektenfamilie der Phasmiden III. Phasmida Anareolatae (Phibalosomini, Acrophyllini, Necrosciini) (in BrunnervonWattenwyl, K. and Redtenbacher, J. 1906-1908). Pp. 339-589, pls. 16-27. W. Engelmann, Leipzig.
Rumbucher K. 1974. Zwei interessante Phasmiden-Gynander. Entomologische Zeitschrift 84: 177-178.
Sellick J. 1997. The range of egg capsule morphology within the Phasmatodea and its relevance to the taxonomy of the order. Italian Journal of Zoology 64: 97-104.
Stål C. 1875. Recensio Orthopterorum. Revue critique des Orthoptères décrits par Linné, de Geer etThunberg. Vol. 3. P. A. Norstedt & Söner, Stockholm.
Vickery V. R. 1983. Catalogue of Australian Stick Insects (Phasmida, Phasmatodea, Phasmatoptera, or Cheleutoptera). CSIRO Australia Division of Entomology Technical Paper. No. 20:1-19.
Westwood J. O. 1859. Catalogue of Orthopterous Insects in the Collection of the British Museum. Part 1, Phasmidae. British Museum, London.
Westwood J. O. 1874. Thesaurus entomologicus oxoniensis, or Illustrations of new, rare and interesting Insects etc., presented to the University of Oxford by the Rev. F. W. Hope. McMillan & Co, London.
Windsor D. M., Trapnell D. W. and Amat G. 1996. The egg capitulum
of a neotropical walkingstick Calynda bicuspis, induces aboveground
egg dispersal by the ponerine ant Ectatomma ruidum. J. Insect
Behav. 9: 353-367.